Winter Olympic ice sports are possible due to this one fact: Ice is slippery.
The low friction of ice is why speedskaters can reach 35 mph, why figure skaters can twirl in dizzying circles, and why a 40-pound curling stone can glide and accomplish whatever the heck the point of curling is.
But for much of the past two centuries, scientists have struggled to explain why, exactly, ice is slippery — and why skates can glide atop it so well.
One obstacle: Skates on ice are surprisingly hard to study. You can’t see what’s happening when a blade is cutting through the ice because the blade obscures the view. And the ice layers that skates glide on are microscopically thin.
So scientists have to rely on their knowledge of physics and chemistry for an explanation. They’ve come up with a few overlapping ones that each elucidate a fascinating property of ice.
First off, a refresher: What is ice?

Ice is solid water. You know that. But what happens when it becomes a solid makes this substance unusual and fascinating.
For most substances in the universe, the solid phase is denser than the liquid phase. When a material is cooled enough to form solids, its molecules get bonded in tight arrays. But ice is different. When it drops below 32°F, the special hydrogen bonds that link water molecules together force additional space between the water molecules when they freeze.


The ice used in rinks at the Olympics isn’t all that different from the ice that comes out of your home freezer. It’s just fussed over a bit more.
As Smithsonian Magazine explains in an excellent feature, Olympic ice is purified water, sprayed on rinks one layer at a time to create surfaces of flawless consistency. The thickness and temperature of the ice in the Olympics depends on the sport. Figure skaters prefer ice set close to the melting point at 25°F for extra grip and control. Hockey players like a colder, harder, and ultimately faster surface.
Solid ice is actually less dense than liquid water (this is why icebergs float in the ocean). And for scientists, this was a clue to figuring out why ice is so darn slippery.
Hypothesis 1: Pressure melts ice. (This is mostly wrong. But still interesting.)
Since the 19th century, the most common answer to the question of “why is ice slippery” has been “because ice melts under pressure.”
This idea draws from the work of James Thompson, who in the 1850s worked out the math that describes a very strange property of ice: That is, under high pressure, ice turns back into water. This is due to the fact, again, that solid ice is less dense than water. If you squeeze ice, it becomes less stable and melts.
You can demonstrate this effect with a very simple experiment. Take a length of wire and tie a weight to each end. Then lay the wire across a large block of ice. The pressure of the wire will cut a clean line through the ice (which will freeze back together once the wire passes through, a process called “regelation”). See that happen here:
It’s tempting to think this is how ice skates work — that the pressure exerted by the thin blade on the ice melts enough water to reduce the friction and allow for gliding.
But here’s the problem: “You’d have to be an incredibly massive person in order to melt ice sufficiently to be able to skate at any reasonable temperature,” David Limmer, a theoretical chemistry professor at UC Berkeley, says.
As the New York Times’s Kenneth Chang has explained, a 150-pound person standing on blades would only lower the melting point of ice from 32°F to 31.97° F., while ice rinks for figure skating are commonly kept around 24°F. Simply put: Skaters can’t exert enough pressure to melt the ice.
“So while the basic idea is correct — you can melt ice by pressurizing it — the numbers don’t work out at all,” Limmer says. (One paper explains if the ice is a very cold -4°F, it would take a pressure of 39,680 psi to melt enough to allow for skating. That’s more than double the pressure found at the bottom of the ocean! And yet it’s still possible to skate on ice that cold.)
Also, pressure melting doesn’t happen instantaneously, as you can see in the video above. “So it’s inconceivable that in the millisecond that a skater spends when on a certain spot on the ice, one can simply, by pressure, melt a layer of water,” Hans van Leeuwen, a retired professor of theoretical physics who has recently published a mathematical explanation of ice skating, says.
Hypothesis 2: Friction melts the ice. (Getting warmer. But it doesn’t explain everything.)
So the pressure of a thin blade on ice cannot explain why skates glide. But what about friction? Can’t the sliding motion of ice skates on the surface generate enough heat to melt the ice?
This is definitely part of the answer, but it doesn’t explain why ice is so unusually slippery to begin with. Anyone who’s walked on a slick sidewalk knows you can slip on ice the second your foot touches it. And that’s not enough time to generate the friction necessary to melt a film of water.
Friction “is a second-order effect” in the ice skating problem, Limmer explains. Friction helps us understand why ice skates can glide faster and faster when moving, but not why they can get started in the first place.
Hypothesis 3: There’s a very small layer of liquid water on top of ice. (This is key.)
A few years before James Thompson explained why pressure melts ice, physicist Michael Faraday discovered another fascinating property of ice: the thin, liquid layer on its surface. His experiment was so simple you can do it at home.
Take two ice cubes in your freezer, and very quickly — so as to not heat any part of them to their melting point — stack them on top of each another.
Come back a few hours later. They’re stuck together.
Faraday (correctly) guessed that the ice cubes stick together because of the liquid layer surrounding them. When these liquid layers meet, they freeze together.
This very thin liquid layer also makes ice extra smooth. But Faraday couldn’t prove his hypothesis at the time. The science of atoms and molecules was not yet available to aid in the explanation.
In 1987, scientists verified the existence of this “quasi-liquid” layer with X-ray imaging. And it’s super, super thin. The best estimates find its thickness at -1°C (30.2°F) is between 1 nanometer and 94 nanometers. That’s about 1,000 times smaller than a bacteria. More recently, scientists have actually seen the liquid surface using extremely sensitive microscopes.
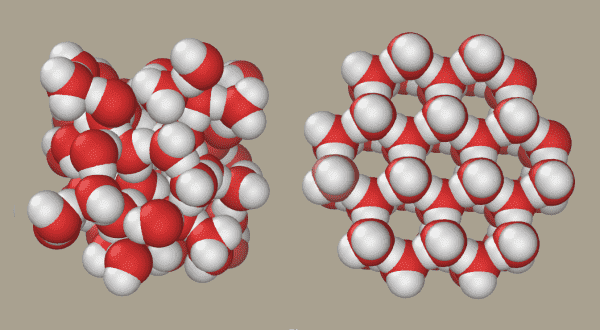
Here’s a diagram showing what’s happening on a molecular scale.
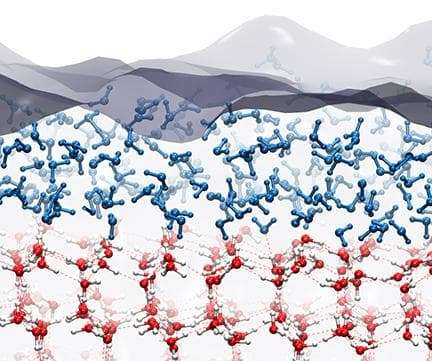

When water is frozen, the individual water molecules grab each other via hydrogen bonds, holding one another in place in a crystalline structure, as you can see in the lower half of the figure. But the molecules on the surface have fewer other molecules to cling to, which makes them more disorganized — and ultimately makes the ice slippery.
Putting it all together



So what happens when an ice skate made from aluminum or steel touches the ice?
Van Leeuwen explains that the tiny liquid layer is the reason why skates can start moving instantaneously on ice. And as the blades move faster and faster through the ice, more friction is generated, which melts more water. As the skater propels forward, she physically plows through the ice, deforming it. This causes more friction, and more melting. All this allows skaters to glide, hydroplane-like, on a thin, thin film of water in a channel they carve. And this all happens in an instant.
Again, all this would be a very hard thing to see firsthand in an experiment. “The thickness of the layer of water is so small that you can’t distinguish it from the ice,” Van Leeuwen says. So it stands as a hypothesis for now.
But interestingly, Van Leeuwen estimates it would be very hard to skate at temperatures below -30°C. Even though there would still be a tiny liquid layer on the ice, it would take too much friction to generate enough heat to melt anything else. Also, below this temperature, the tiny liquid layer on top of ice becomes harder and harder to detect. It would be like skating on gravel. (Though why in the world you would want to go skating at -22°F is beyond me.)
What else can we skate on?
Okay. Case closed. But I was left wondering: Are there any other surfaces we could skate on? As Limmer explains, “basically all solids” will form a tiny liquid layer “when they are close to their melting temperature.”
Mercury freezes at -37.89°F. It would take far too much energy to keep a rink of mercury that cold. Plus, mercury is a potent neurotoxin.
What about gallium? It’s a metal that melts at 85.58°F — a little hot for a skating rink. But just imagine skaters doing triple axels on a silvery mirror gallium surface. “That sounds like a wonderful idea,” Limmer says.
Though solid gallium would be more slippery near its melting point, would it be slippery enough for ice skating? Or easy enough to plow through? There’s only one way to find out.
Sourse: vox.com