
With e-cigarette use now epidemic among teens and cigarettes still the leading cause of preventable death in the United States, the Food and Drug Administration is planning two major moves this week: restricting the sale of flavored e-cigarettes across the US, and banning menthol cigarettes, according to multiple news outlets.
The menthol ban will require new regulation — and will take years to go into effect — but it would have a greater health impact: Researchers have long known menthol cigarettes are more attractive to youth and harder to quit than regular cigarettes. They’ve also been heavily marketed at — and are especially popular among — black smokers.
“I don’t think the tobacco industry should be marketing products that, if not directed at kids, make it exceptionally easy to get kids started on combustible cigarettes,” FDA Commissioner Scott Gottlieb said at a public event on Tuesday, in which he also hinted that the new restrictions could include flavored cigars.
The e-cigarette ban does not require new regulation and could happen immediately. It is likely a response to new data showing a 75 percent increase in the use of vaping devices among high schools since 2017, as well as evidence that teens are quickly adopting products like Juul, a vape device that burns pods with flavors like Creme Brûlée.
First reported by the Washington Post, the new FDA vape restrictions are expected to include a ban on the sale of flavored cartridge-based e-cigarettes in convenience stores and gas stations across the US, as well as age-verification requirements for online sales to minors. Tobacco and e-cigarette stores for adults will still carry these products, according to the Post, and mint and menthol e-cigarettes will be exempt from the restrictions.
By curtailing access to vape and cigarette products that appeal to youth, the FDA is aiming to drive down vaping and smoking rates in minors without adding any barriers for established smokers who want to move to vaping. But with teen vaping already widespread in America, that might be easier said than done.
Gottlieb’s e-cigarettes restrictions follow the rise in teen vaping
Tobacco remains a major killer in the US. But when the FDA banned flavor claims on regular cigarettes, Congress exempted menthol in 2009, to the dismay of health advocates. So the proposed changes would close the cigarette gap but leave mint and menthol e-cigarettes relatively easily available.
Since his first days on the job, Gottlieb appears to have struggled with how to address e-cigarettes in that context.
In July 2017, the FDA delayed the compliance deadline for the regulation of e-cigarette products to 2022. This gave the industry five more years to file public health applications that show that their products are safe alternatives to conventional cigarettes and that they weren’t unduly targeting minors.
Gottlieb positioned the delay as a way to give manufacturers time to get in step with the new laws while ensuring smokers had access to cigarette alternatives that could save their lives. But some public health advocates viewed it another way: as a giveaway for the vaping industry, and a chance for e-cigarette makers to further expand their market share among kids at a time when e-cigarette use by teens has eclipsed conventional cigarette use.
“In this world, a delay of [five] years is a lifetime,” Matt Myers, president of the Campaign for Tobacco-Free Kids, told Vox last spring. “And the data seems to indicate this product is being used by kids all across the country.”
For these reasons, health groups sued the FDA over the delay and sent a letter to Gottlieb asking the FDA to begin regulating e-cigarette products like other cigarettes immediately.
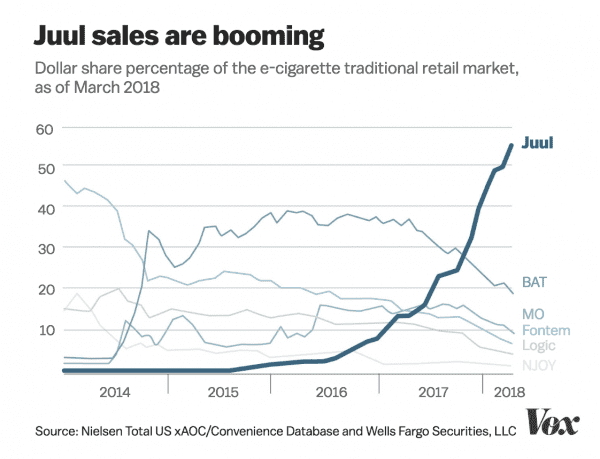
In 2017, the e-cigarette market grew by 40 percent. Juul has been driving a big portion of that growth.
Meanwhile, the e-cigarette market has continued to grow. In 2017, the US market expanded by 40 percent, to $1.16 billion, with a lot of that growth driven by Juul.
As of March, Juul made up more than half of all e-cigarette retail market sales in the US, according to Nielsen data. Considering it has only been on the market since 2015, and there are hundreds of other devices available to consumers, Juul’s market share is staggering.
More recently, the FDA has been taking action against Juul and teen e-cigarette use — but has stopped short of changing the compliance deadline. In April, the FDA took the unusual step of demanding Juul Labs submit documents about its marketing and research and what it knows about Juul use among young people. The move was part of the FDA’s new Youth Tobacco Prevention Plan.
In May, the agency followed up by sending requests for information to four other e-cigarette makers that also appear to be marketing to young people.
Last September, FDA announced it has again sent warning letters to Juul Labs and the manufacturers of other vaping devices, giving them 60 days to come up with plans that minimize their products’ appeal to minors. It’s also warned more than 1,000 retailers accused of illegally selling e-cigarettes to minors to stop.
Juul Labs, the maker of Juul, this week appeared to be preempting the FDA’s crackdown, with an announcement it would stop selling its fruit and dessert-flavored products in more than 90,000 brick-and-mortar retail stores, and halt its marketing on Facebook and Instagram.
But the FDA is expected to use the regulatory authority it already has over e-cigarettes to impose the new restrictions. Along with the menthol ban, the agency is striking a good balance, public health researchers said.
“Whether you’re heavy or light smoker, on the harm reduction it doesn’t make sense to have the far more harmful products more appealing in terms of flavors or more accessible in terms of retail availability,” said University of Waterloo public health researcher David Hammond.
The new restrictions will do both: crack down on access to vaping, which can lead to smoking, while also “tightening screws on smoking,” Hammond added.
“This is a thoughtful approach and will help reduce the current epidemic,” said David Liddell Ashley, the previous director of the office of science in the Center for Tobacco Products at FDA. But, he added, “I do not think it will completely solve the likely increase in cigarette smoking, which will result from the use of e-cigarettes by youth.”
That would require a number of extra steps — like engineering products so they’re less addictive and cracking down on marketing. And the FDA has stalled on both these fronts.
For more on Juul and e-cigarettes, read our explainer, listen to the May 3, 2018, episode of Today, Explained, and watch our Vox video here.
Sourse: vox.com






