
Lyme has quickly become one of the most common infectious diseases in America, with many as 300,000 people infected every year. And public health officials fear the bacterial infection, which jumps from ticks to humans, will only spread farther and faster as climate change makes more parts of the US habitable for ticks.
Lyme can be treated with antibiotics. And there are many ways to prevent tick bites. But there’s no vaccine available if you want extra protection against the disease (unless you’re a dog).
Yet in the late 1990s and early 2000s, a vaccine called LYMErix was sold to prevent between 76 and 92 percent of infections. Hundreds of thousands of people got it — until vaccine fear knocked it off the market.
The LYMErix story is worth retelling today. It’s a stark reminder of how anti-vaccine mania of the past few decades is leaving us all more susceptible to disease.
The Lyme vaccine was effective
Lyme first appeared in the US seemingly out of nowhere, spreading between ticks and people in Connecticut.
By the 1990s, it was possible to be infected with Lyme from a tick bite in much of the northeastern US — and there were around 15,000 confirmed cases a year. (Today, there are more than 35,000 confirmed or probable cases of Lyme each year and many more cases that go completely unreported.)
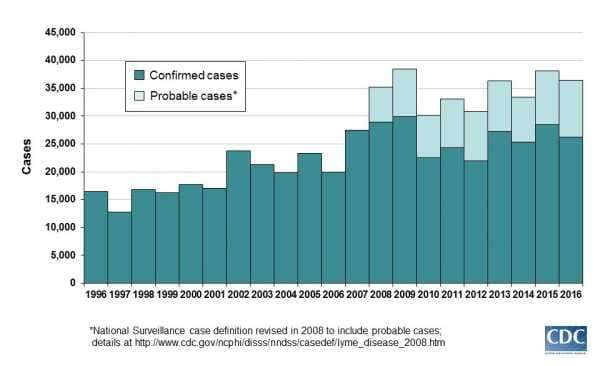
Recognizing the increasing public health hazard, the drug manufacturer SmithKline Beecham (now called GlaxoSmithKline) developed a vaccine that targeted the outer protein of the bacteria that causes Lyme. The Food and Drug Administration approved it in 1998.
The vaccine worked by targeting the bacteria while it was still inside the tick’s body, the website History of Vaccines explains. The bacteria would be neutralized before the tick ever had the chance to transfer the bacteria into the human body.
LYMErix wasn’t a perfect vaccine, as Gregory Poland, a Mayo Clinic vaccine researcher, explained in a 2011 retrospective in the journal Clinical Infectious Diseases. It required three doses over the course of the year, and was not approved for people under age 15. It was optional, and doctors had a hard time assessing whom to recommend it to (there were few maps of Lyme-carrying ticks’ range at the time). And the vaccine only protected against the North American strain of Lyme. Finally, it was somewhat expensive at $50 a dose, and it was not universally covered by health insurance.
But it was effective, preventing Lyme in up to 90 percent of the people who were vaccinated will all three doses, with few side effects. And at first, the vaccine was pretty popular; about 1.5 million doses were injected before 2000.
LYMErix debuted near the beginning of anti-vaccine mania

LYMErix had the misfortune of being approved the same year some people were becoming suspicious of vaccines in the United States. In 1998, the journal Lancet published a now-retracted study that (falsely) claimed the measles, mumps, and rubella vaccine (MMR) was linked to autism, and the modern anti-vax movement was born.
At the same time, a few members of the FDA panel that approved LYMErix had voiced a theoretical concern that the drug could cause an autoimmune reaction leading to arthritis. The idea was that as the immune system learned to attack the protein that covered the Lyme bacteria, it could overreact and start to attack healthy tissue in the body. This side effect didn’t occur in the clinical trial. It was just a hypothetical possibility.
The FDA panel eventually unanimously approved the drug, but the fear of an autoimmune reaction trickled down to the public.
What happened next was a perfect storm to drive the product from the market. A 2000 study found the vaccine contributed to autoimmune arthritis in hamsters. Other research posited (but didn’t prove) that it was possible some people were more genetically predisposed to develop this type of autoimmune response in reaction to the vaccine.
Sure enough, some LYMErix recipients soon began to complain publicly that the drug was causing them to develop joint pain. National news media were reporting on the concerns, casting them in a harrowing light. In the 2000, ABC news told the story of a man who fell ill with a “fever and an intense, hellish pain” after taking the vaccine.
The FDA looked into the claims but never found a connection between the vaccine and arthritis. By 2001, 1.4 million does of the vaccine had been distributed, but the FDA’s Vaccine Adverse Events Reporting System only picked up on 59 reports of arthritis.
“The arthritis incidence in the patients receiving Lyme vaccine occurred at the same rate as the background in unvaccinated individuals,” a 2007 paper in Epidemiology and Infection explains.
Overall FDA’s VAERS only picked up on 905 reports of any adverse side effects at all — a tiny amount compared to the number of people who had gotten the shots.
The vaccine was pulled from the market, despite evidence finding it was safe
But it was too late. Already, there was “significant media coverage, sensationalism, the development of anti-Lyme vaccine groups … who urged withdrawal of the vaccine from the market,” Poland explained in his 2011 article. A class-action lawsuit targeted SmithKline Beecham, claiming the company did not do enough to warn people of potential autoimmune side effects.
The FDA continued to follow up with an additional drug safety trial to try to settle the matter for the public. The trial was supposed to last four years. But sales of LYMErix had plummeted “from about 1.5 million doses in 1999 to a projected 10,000 doses in 2002,” the National Institute of Allergy and Infectious Diseases explains on its website.
So the manufacturer pulled it from the market, despite the fact that early data from the additional safety trial found “no differences in any significant adverse reactions noted between control subjects and vaccinated persons,” Poland writes.
Concerning side effects sometimes do emerge after a drug comes on the market. But you need hard data to establish them. And the FDA’s investigations into LYMErix never found any evidence of autoimmune side effects.
“Although studies never adequately substantiated the safety concerns associated with LYMErix,” the Epidemiology and Infection article states, “the decline in public tolerance for risk and uncertainty combined with the relatively low morbidity of Lyme disease contributed to the inability of the vaccine to find a market niche.”
In 2000s, Lyme still didn’t infect that many people, and the public was more concerned about the Lyme vaccine than the disease itself. But now infections rates are rising and we’re left without a crucial tool to stop its spread.
Where are we now?
As Julia Belluz reported at Vox, Lyme cases tripled between 2004 and 2016, spread by an increased number of infected ticks. It’s now the most common vector-borne (i.e., transmitted by an insect) disease in the United States. And climate change seems to be partly to blame: As temperatures warm, a greater proportion of the US becomes hospitable to the ticks. Overall, vector-spread diseases like chikungunya, Zika, and West Nile are spreading faster than ever.
And still, if you wanted to protect yourself with a Lyme disease vaccine, you couldn’t get one. As Belluz explained, prevention efforts currently focus on avoiding tick bites. That means covering up exposed skin when spending time in wooded areas, using insect repellent, and checking your body for ticks (and removing them) after you’ve spent time outdoors in tick-laden areas.
WBUR in Boston reports there have been some small efforts to revive LYMErix (its patent has now expired), but the pharmaceutical industry has lost interest in it, and grassroots efforts have gone unfunded. The Lyme vaccine for dogs works in a similar manner to LYMErix. But while it does help control the spread of the disease, it doesn’t make up for the lack of a vaccine in humans.
“Low demand for the vaccine and its subsequent withdrawal from the market represent a loss of a powerful tool for Lyme disease prevention,” the authors of the Epidemiology and Infection article state. For many, symptoms last months, leading to painful arthritis, heart problems, and nerve pain. Though Lyme is treatable, it needs to be diagnosed early for people to avoid its worst symptoms. A vaccine would provide a greater margin of error if a tick bite goes unnoticed.
Unscientific anti-vaccine movements leave us all more unsafe down the line. We see examples of this in the news all the time. Diseases long controlled by vaccines, like measles, are now starting to pop back up in concerning numbers. In Japan, vaccination rates for HPV vaccine plummeted in recent years due to fearmongering.
Vaccines can be a hard sell because people need to take them when they’re healthy, and no vaccine has zero risk of side effects. But when we take a vaccine, we’re not just protecting ourselves — we’re protecting those around us, and ensuring a less infected future. The LYMErix vaccine was optional, and anti-vaccine fears have left millions without the option to take it at all.
A French company is developing a new Lyme vaccine, New Scientist reports. It would protect against the different strains of Lyme that circulate worldwide, but it’s just getting out of Phase I safety trials, which means it would be many years before it arrives on the market, if proven safe.
We can’t count on having a vaccine anytime soon. But we can count on more ticks coming our way.
Sourse: vox.com






