Over PLN 1.14 billion will go to biomedical research from the National Reconstruction Plan. In January and February, the Medical Research Agency resolved all competitions entrusted to it by the Ministry of Health, including recently for research in the area of innovative therapies, medicines of the future and drug safety. For the entire biomedical sector, the coming months may be a breakthrough time, because the funds from the KPO must be used by mid-2026. This acceleration is a benefit not only for companies and scientific institutions, but also for patients and the economy.
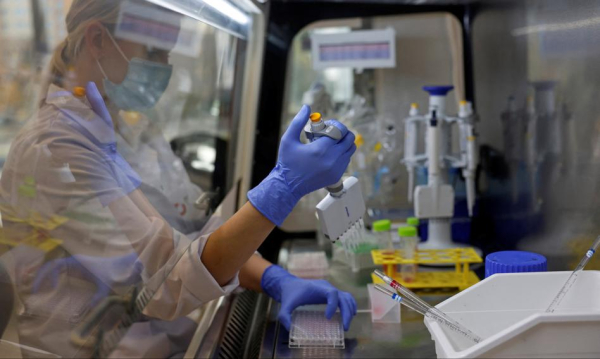
– Since the beginning of its activity, i.e. for five years, the Medical Research Agency has allocated PLN 4.5 billion for 324 projects in the biomedical area, of which PLN 922 million went to Polish entrepreneurs who develop new technologies, both in the area of medicinal products and medical devices. As part of the National Reconstruction Plan, we are adding approximately PLN 1.14 billion, which are to strengthen research and development in the area of medicinal products and medical devices . But we also support Clinical Research Support Centers, i.e. such systemic and comprehensive solutions that are to increase the competitiveness of the Polish clinical research market – Ireneusz Staroń, Deputy President for Research Financing at the Medical Research Agency, tells the Newseria agency.
In January, ABM announced the results of a competition for entrepreneurs to conduct research in the area of drug safety, innovative therapies and medicines of the future. 22 projects were selected to receive funding for a total amount of over PLN 112 million. This is another competition implemented by the agency using funds from the National Reconstruction Plan. The projects recommended for funding include drugs for respiratory diseases, targeted anticancer therapy and viper venom antitoxin. The thematic range of submitted and awarded projects was very wide, and most of them concerned oncology.
– For example, we have heard that Ryvu's innovative projects in the area of biological products are in the second phase of clinical trials. I think that as a country we have an appetite for these Polish innovations – for example in the area of biological drugs and cell therapies – to be implemented on the market. In the perspective of 5-10 years I would like to see them available, achieving global success and reaching patients in Poland and in many countries around the world, including the largest market, i.e. the United States, where revenues and margins are the highest – emphasizes the deputy president of ABM.
As ABM representatives emphasize, this year is to be a breakthrough for the clinical and biomedical research sector thanks to the additional PLN 1.14 billion from the KPO. Some of these funds will go to pharmaceutical and biotechnology entrepreneurs as real support in conducting research on innovative therapies. This is to contribute not only to accelerating work and strengthening the company's competitiveness, but also to increasing the country's drug safety and stimulating the Polish economy.
– We should definitely invest as much as possible in this area, because we want to build an economy based on knowledge and new technologies. We can translate relatively low funds invested in this industry into quite a large gross value added. We are talking about a macroeconomic indicator that translates into building gross domestic product. The pharmaceutical industry alone provides around 5 billion euros annually. If we add all other derivative sectors in the area of clinical trials, for example, we are talking about a powerful pillar of economic growth – assesses Ireneusz Staroń.
In January, ABM also decided on another competition with the KPO for scientific units to carry out applied research in the biomedical area. Recommendations for funding were received by 57 projects, for a total amount of almost PLN 447 million. These include monitoring the effects of treatment of cervical cancer, oropharyngeal cancer or anogenital cancer, research on the genetic causes of hearing loss and a test enabling earlier diagnosis of ovarian cancer. This competition enjoyed record interest – 224 applications were submitted, three times more than in other competitions previously carried out by ABM.
– The interest in KPO programs is record-breaking due to the huge demand, both from entrepreneurs and scientific units, for the implementation of research and development projects in the biomedical area – says Karolina Nowak, Ph.D., director of the Department of Innovation and International Cooperation at the Medical Research Agency. – As part of the projects to date, the agency finances 44 commercial projects in the area of new innovative products, generic products, new pharmaceutical forms, but also from funds from the National Reconstruction Plan, it has begun financing biomedical projects for scientific units. As part of the competition for scientific units, ABM will co-finance 57 projects that will strengthen the biomedical area and help Polish scientists develop their application solutions, i.e. those focused on commercialization.
Funding from the KPO is a great chance to accelerate the development of a given project, but also a big challenge related to time constraints. These funds must be used by mid-2026, which seems to be too short a perspective for biomedical research.
– That is why the Medical Research Agency came up with the idea that projects will be financed only at a certain stage of their implementation – explains Karolina Nowak, Ph.D. – We are at the stage of signing agreements so that our beneficiaries can start implementing their projects as soon as possible. The pace was fast, the substantive assessment took us some time due to the very large number of applications, as well as because it was performed by external experts to ensure full transparency of the entire process. As part of the competition for entrepreneurs, we also had an additional financial assessment of companies, but everything was done according to plan.
At the beginning of January, the competition for the creation and development of Clinical Research Support Centers, also organized as part of the KPO, was also resolved. PLN 200 million, i.e. the total amount of support for medical units, will allow for the launch of 12 new facilities in eight provinces. Thanks to this, the network of CWBK, i.e. centers coordinating clinical trials and supporting the launch of non-commercial clinical trials, will expand to 33.





