Pfizer and BioNTech have launched a clinical trial on a vaccine targeting both COVID-19 and influenza, the companies announced Thursday.
The phase 1 trial is being done in the United States with 180 participants between the ages of 18 and 64, with the first participant dosed this week, the companies said. The follow-up period for each participant will be six months.
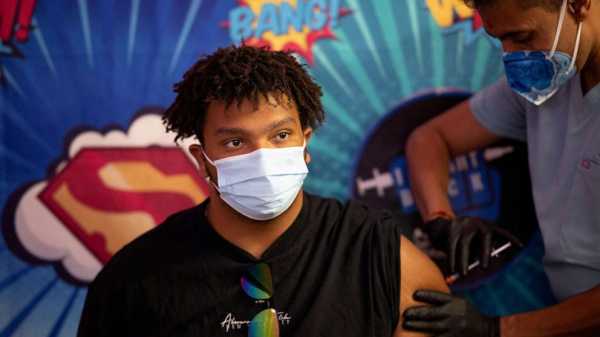
In this Sept. 8, 2022, file photo, a teenager receives the Pfizer-BioNTech COVID-19 booster vaccine targeting BA.4 and BA.5 Omicron sub variants at Skippack Pharmacy in Schwenksville, Penn.Hannah Beier/Reuters
"By combining both indications in one vaccine approach, we aim to provide individuals with an efficient way to receive immunization against two severe respiratory diseases with evolving viruses that require vaccine adaptation," Dr. Ugur Sahin, CEO and co-founder of BioNTech, said in a statement.
The combination vaccine is based on the currently available bivalent COVID-19 booster and a quadrivalent mRNA flu vaccine, which is designed to protect against four different flu viruses.
The phase 1 trial will test for safety, immune response and optimal dose level of the combination vaccine, before moving on to larger trials. The data will also provide insight into the potential of mRNA vaccines to address more than one pathogen, Sahin said.
MORE: Is the US facing a potential 'tripledemic' of flu, RSV and COVID-19?
Annaliesa Anderson, senior vice president and chief scientific officer of vaccine research and development for Pfizer, called this an "exciting step in our ongoing journey with BioNTech as we collectively look to transform the prevention of infectious diseases around the world."
"Even with existing seasonal influenza vaccines, the burden of this virus is severe across the world causing thousands of deaths and hospitalizations every year," she said in a statement.
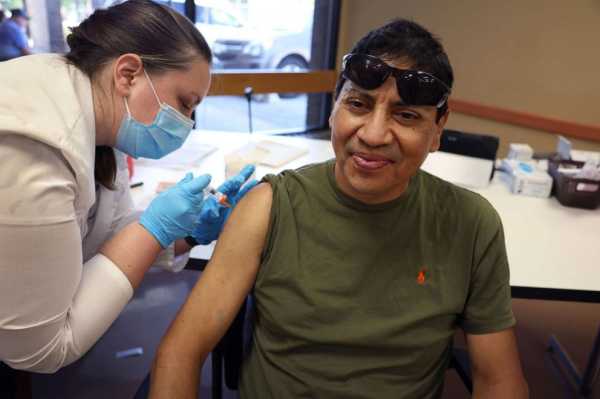
In this Sept. 9, 2022, file photo, a man gets an influenza vaccine from a pharmacist during an event hosted by the Chicago Department of Public Health at the Southwest Senior Center in Chicago.Scott Olson/Getty Images
Studies indicate COVID-19 vaccine efficacy fades over time, though it's not clear if every American will need an annual COVID-19 booster. As scientists continue to assess the need, several companies are at work on creating a single injection each fall that protects against both seasonal flu and COVID-19.
MORE: Scientists are working on combo flu and COVID-19 shot, but don't expect one this fall
In addition to Pfizer, pharmaceutical companies Moderna and Novavax have announced plans to work on a combo shot.
Moderna said it anticipates starting clinical trials on a single-dose vaccine that combines a booster against COVID-19 and a booster against flu by the end of the year, with hopes of the vaccine being available for the 2023 season.
"We believe this is a very large opportunity that is ahead of us, if we could bring to market a high efficacy pan-respiratory annual booster," Moderna COE Stéphane Bancel said during the Sept. 9 investor meeting.
Last year, Novavax enrolled people in a Phase 1/2 study to evaluate the safety, tolerability and immune response of a combination vaccine using the company's seasonal influenza and COVID-19 vaccines. A phase 2 confirmation trial is expected to begin later this year, the company said in October.
Sourse: abcnews.go.com






