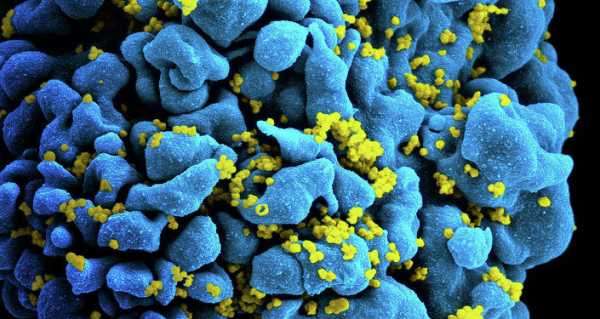
US biotechnology firm American Gene Technologies (AGT) on Tuesday received approval from the US Food and Drug Administration (FDA) to start a phase-one clinical trial of a potential cure for the human immunodeficiency virus (HIV), which causes acquired immunodeficiency syndrome (AIDS).
The trial, which will take place in Baltimore, Maryland, and Washington, DC, is expected to begin enrollment in September and will test the safety of AGT103-T, a single-dose gene therapy developed to cure HIV.
“In HIV disease, the HIV-specific CD4 T cells are activated soon after infection similar to other viral diseases. However, HIV targets these cells for destruction because this insidious virus binds to the same CD4 molecule that identifies the helper T cell subset,” AGT explained on its website, noting that the HIV-specific CD4 T cells become compromised after an HIV infection because they fall below the necessary levels required for effective immune function.
The new, potential treatment thus delivers HIV-specific CD4 T cells to people infected with the virus. By restoring the missing cells, the drug is intended to repair the immune system damage caused by HIV.
The potential cure was developed in collaboration with the US National Institute of Allergy and Infectious Diseases. AGT hopes to report initial findings from the study before the end of the year.
According to UNAIDS, around 38 million people across the world in 2019 were living with HIV. An estimated 1.7 million people worldwide acquired the virus last year.
Sourse: sputniknews.com






